Just months after dangerous abortion drugs went on the market in Canada, the government already is expanding their use.
Health Canada dropped important safety measures on the abortion drugs Tuesday in a move that could lead to more unborn babies’ abortion deaths and more injured mothers. The government announced Tuesday that the abortion drugs now may be used up to nine weeks of pregnancy, instead of seven, The Canadian Press reports. According to the new regulations, women no longer need to take the drugs under the supervision of a doctor either, despite potentially life-threatening risks.
The CBC reports the drugs now will be available by prescription and dispensed by pharmacists, and doctors no longer will be required to take a special course before prescribing them. Women also will not have to give written consent to take the drugs, the report states.
Unlike in the United States, the abortion drugs mifegymiso only became available on the market this year in Canada. Mifegymiso is a two-drug abortion method known in the U.S. as mifepristone, or RU-486, and misoprostol. The abortion drugs are responsible for killing millions of unborn babies in the United States and at least 14 women, since they were approved during the Clinton administration.
Health Canada said the changes come after the department “received and rigorously reviewed new scientific evidence submitted by the drug sponsor.” The department has also “undertaken a thorough review of new and existing scientific literature on the safe use and effectiveness of Mifegymiso.”
Here’s more from the Canadian Press:
Health Canada said women will no longer be required to provide written consent to take Mifegymiso, nor will health professionals need to register with the drug’s distributor, Celopharma, to prescribe or dispense it.
While medical professionals should have appropriate knowledge about the drug before prescribing it, the federal department said they will no longer be required to first complete a formal education program in its use.
However, one carry-over from the previous version of Health Canada’s prescribing guidelines is the mandatory requirement that women have an ultrasound to ensure they don’t have an ectopic pregnancy — one outside the uterus — and an assessment of the length of gestation.
Abortion activists are complaining about the government’s decision to keep the ultrasound requirement, despite the loosening of other regulations.
Follow LifeNews.com on Instagram for pro-life pictures and the latest pro-life news.
“There is decades worth of evidence from use in over 60 countries proving how safe and effective this medication is, yet Mifegymiso is more regulated than controlled substances in Canada,” Sandeep Prasad, executive director of the pro-abortion group Action Canada, said in a statement.
Prasad complained that the ultrasound requirement could mean some women in rural areas may have a hard time getting an abortion.
It is yet more proof that abortion activists care more about women getting abortions than women’s safety or their unborn babies’ lives.
In 2012, a U.S. Food and Drug Administration report indicated that 14 women in the United States alone died from using the mifepristone abortion drug and 2,207 women were injured by it.
The dangerous abortion drug has claimed the lives of more than 2 million unborn children in the United States since its approval at the end of the Clinton administration. In addition to the number of unborn children whose lives have been destroyed, women have suffered as well, as a Planned Parenthood study admits at least one woman is seriously injured from the abortion pill daily.
Because of the high failure rate and the risks involved with the abortion drugs in later pregnancies, the U.S. Food and Drug Administration limited approval for use only in the first 49 days from the start of a woman’s last menstrual period. The Canadian government also approved the drug for the first seven weeks of pregnancy initially.
However, last year under pro-abortion President Barack Obama’s administration, the FDA changed its guidelines for the drug, allowing it to be used later in pregnancy and in smaller doses.
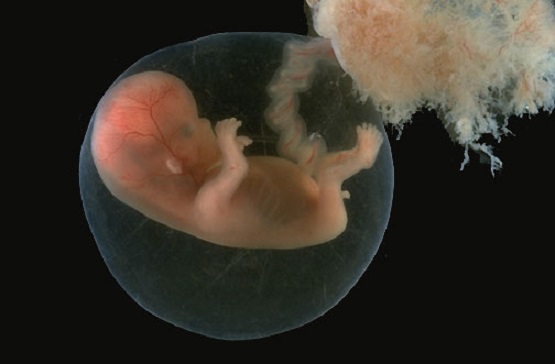
A 9-week-old unborn baby appears below.